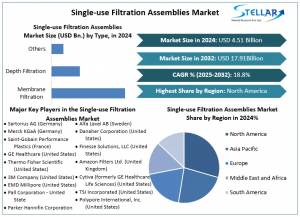
Single-use Filtration Assemblies Market Size Projected to Surpass USD 17.91 Billion by 2032 To Forecast 2025-2032
Single-use Filtration Assemblies revenue is expected to grow at a CAGR of 18.8% from 2025 to 2032, reaching nearly USD 17.91 Bn. by 2032.
The Single-use Filtration Assemblies Market is projected to grow at a CAGR of approximately 18.8% over the forecast period. The Single-use Filtration Assemblies Market was valued at USD 4.51 billion in 2024 and is expected to reach USD 17.91 billion by 2032. Growing biopharma industries, better control of contamination, cutting costs, tech upgrades, more chronic diseases, rules to follow, and more CMOs push the Single-use Filtration Assemblies Market all around the world.
Single-use Filtration Assemblies Market Overview
The Single-use Filtration Assemblies Market is getting big fast due to more want in drug-making, shots, and clean making. These units give use-and-throw, ready-clean filter fixes that cut out washing, drop dirt risk, and make work faster. Main pushes are the up in life drugs, rules need for dirt block, tech steps up, and more deal-making groups. The market wins from being able to change, saving cost, and quick use of one-use tech, making them key in new drug making and drug work.
To know the most attractive segments, click here for a free sample of the report: https://www.stellarmr.com/report/req_sample/Single-use-Filtration-Assemblies-Market/1739
Single-use Filtration Assemblies Market Dynamics
Drivers
Need for Contamination Control and Sterility
Rules set by groups call for full clean work in drug making to keep it safe. One-time use filters cut out washing and using again, which lowers the chance of mix-ups. New steps up have better screens, tight-sealed systems, quick bug checks, and rule syncing, making the clean promise stronger. New ideas also look at earth-friendly stuff, mixing dirt control with care for our world, pushing more use in clean methods.
Rapid Growth of Biopharmaceutical and Vaccine Production
The fast-growing drug making area, like shots and gene fixes, needs clean, changeable, and big-scale filters. One-time use filter setups help quick making, cutting down on dirt risks. COVID-19 sped up shot making, pushing more use. Steps up in build-able sites, smart checks, and green stuff help the market grow more, making one-time use filters key for new drug making and making medicine for one person.
Technological Advancements in Materials and Design
New steps in filter tech, designs, and full set-ups make one-use filters work better, fit better, and easier to use. Changes add smart tools to check in real-time, green stuff, and systems you can change. These better bits up speed, spread uses, and make rule-following easy, pushing many to pick one-use filters in drug and biotech making.
Restrain
High initial costs
Big start costs for one-time use filter kits hold back use, more so for small makers. But, cutting costs, scale ups, mixed lasting-one-time models, and rent offers ease money blocks. Also, help aids and price cuts back new firms, making one-time use filters more within reach despite early money tests, pushing slow but steady market rise.
Innovations and Developments
Technological innovation is a key factor propelling the Single-use Filtration Assemblies Market forward. Notable advancements include:
Advanced Membrane Materials: New plastics like polyether sulfone (PES), polyvinylidene fluoride (PVDF), and freshly made membranes bring better chemical match, more flow, and strong germ hold.
Modular and Pre-sterilized Assemblies: Ready-made, clean units with set links cut down setup time and dirt risk, making quick changes and bendy making easy.
Single-use Filtration Assemblies Market Segmentation
By Type
By Type, the Single-use Filtration Assemblies Market is further segmented into Membrane Filtration, Depth Filtration, and Others. Membrane filtration dominates the single-use filtration assemblies’ market because they are exact, clean, and have full rule okay. New ideas and big needs in biopharma push this growth. Though deep filters rise quick in the first steps, membrane filters stay key for must-be-clean uses. They keep the top spot in market share and use all over the world.
Single-use Filtration Assemblies Market Regional Analysis
North America: North America dominates the single-use filtration assemblies’ market due to its strong drug-making field, high money put in research, strict clean rules from the FDA, top-class set-ups, and big names like Thermo Fisher. More growth and company buys have helped it stay at the top in the area.
Europe: Europe ranks second in the single-use filtration assemblies’ market because it has a strong drug-making field, rules from the EMA, and a big push for keeping things clean. Places like Germany and the UK push this growth. Big names such as Sartorius grow by creating new things and buying other firms.
Asia-Pacific: Asia-Pacific ranks third in single-use filtration assemblies due to quick growth in biopharma, cash help from the government, tech gains, and more making skills in China, India, and South Korea, all driving big market growth.
Recent Developments:
Thermo Fisher Scientific bought Solventum's cleaning and filtering part for about $4.1 billion. This buy makes Thermo Fisher better at one-time use filter systems, matching its plan to grow its biopharma filter group.
AbbVie spent $223 million to make its Singapore Tuas Biomedical Park place bigger, raising biologic product making, helping one-time use system use, and adding more than 100 new jobs in its world making net.
To know the most attractive segments, click here for a free sample of the report: https://www.stellarmr.com/report/req_sample/Single-use-Filtration-Assemblies-Market/1739
Single-use Filtration Assemblies Market Competitive Landscape
The global and regional players in the Single-use Filtration Assemblies Market concentrate on developing and enhancing their capabilities, resulting in fierce competition. Notable players include:
Sartorius AG (Germany)
Merck KGaA (Germany)
Saint-Gobain Performance Plastics (France)
GE Healthcare (United States)
Thermo Fisher Scientific (United States)
3M Company (United States)
EMD Millipore (United States)
Pall Corporation - United State
Parker Hannifin Corporation (United States)
Meissner Filtration Products (United States)
Related Reports:
North America Aesthetic Medical Devices Market: https://www.stellarmr.com/report/north-america-aesthetic-medical-devices-market/2719
Veterinary Excipients Market: https://www.stellarmr.com/report/veterinary-excipients-market/2716
Respiratory and Anaesthesia Disposable Market: https://www.stellarmr.com/report/respiratory-and-anaesthesia-disposable-market/2715
Veterinary Eye Care Market: https://www.stellarmr.com/report/veterinary-eye-care-market/2712
Prothrombin Complex Concentrate Market: https://www.stellarmr.com/report/prothrombin-complex-concentrate-market/2708
About Stellar Market Research:
Stellar Market Research is a multifaceted market research and consulting company with professionals from several industries. Some of the industries we cover include medical devices, pharmaceutical manufacturers, science and engineering, electronic components, industrial equipment, technology and communication, cars and automobiles, chemical products and substances, general merchandise, beverages, personal care, and automated systems. To mention a few, we provide market-verified industry estimations, technical trend analysis, crucial market research, strategic advice, competition analysis, production and demand analysis, and client impact studies.
Contact Stellar Market Research:
S.no.8, h.no. 4-8 Pl.7/4, Kothrud,
Pinnac Memories Fl. No. 3, Kothrud, Pune,
Pune, Maharashtra, 411029
sales@stellarmr.com
Lumawant Godage
Stellar Market Research
+ +91 9607365656
email us here
Visit us on social media:
LinkedIn
Instagram
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Cardiac Registry Support Doubles its Network and Expands into Bariatric Surgery Registry Support Following Acquisition
ECRA Partners with Mauritius Consultant to Pilot Global Early Childhood Quality Accreditation Framework
Michael Fomkin Partners with Jack Canfield and SuccessBooks® as a Co-Author of 'Living Truth'
Więcej ważnych informacji
 Jedynka Newserii
Jedynka Newserii

 Jedynka Newserii
Jedynka Newserii

Konsument
Polacy nie korzystają z hossy trwającej na warszawskiej giełdzie. Na wzrostach zarabiają głównie inwestorzy zagraniczni
Od października 2022 roku na rynkach akcji trwa hossa, nie omija ona także warszawskiej giełdy. Mimo to inwestorzy indywidualni odpowiadają zaledwie za kilkanaście procent inwestycji, a o wzrostach decyduje i na nich zarabia głównie kapitał z zagranicy. Widać to również po napływach i odpływach do i z funduszy inwestycyjnych. Zdaniem Tomasza Koraba, prezesa EQUES Investment TFI, do przekonania Polaków do inwestowania na rodzimej giełdzie potrzeba zysków z akcji, informacji o tych zyskach docierającej do konsumentów oraz czasu.
Polityka
Obowiązek zapełniania magazynów gazu w UE przed sezonem zimowym ma zapewnić bezpieczeństwo dostaw. Wpłynie też na stabilizację cen

Unia Europejska przedłuży przepisy z 2022 roku dotyczące magazynowania gazu. Będą one obowiązywać do końca 2027 roku. Zobowiązują one państwa członkowskie do osiągnięcia określonego poziomu zapełnienia magazynów gazu przed sezonem zimowym. Magazyny gazu pokrywają 30 proc. zapotrzebowania Unii Europejskiej na niego w miesiącach zimowych. Nowe unijne przepisy mają zapewnić stabilne i przystępne cenowo dostawy.
Infrastruktura
Gminy zwlekają z uchwaleniem planów ogólnych zagospodarowania przestrzennego. Może to spowodować przesunięcie terminu ich wejścia w życie

Reforma systemu planowania i zagospodarowania przestrzennego rozpoczęła się we wrześniu 2023 roku wraz z wejściem w życie większości przepisów nowelizacji ustawy z 27 marca 2003 roku. Uwzględniono w niej plany ogólne gminy (POG) – nowe dokumenty planistyczne, za których przygotowanie mają odpowiadać samorządy. Rada Ministrów w kwietniu br. uchwaliła jednak ustawę o zmianie ustawy z 7 lipca 2023 roku, a jej celem jest zmiana terminu obowiązywania studiów uwarunkowań i kierunków zagospodarowania przestrzennego gmin na 30 czerwca 2026 roku. Wskazana data może nie być ostateczna z uwagi na to, że żadna z gmin nie uchwaliła jeszcze POG.
Partner serwisu
Szkolenia

Akademia Newserii
Akademia Newserii to projekt, w ramach którego najlepsi polscy dziennikarze biznesowi, giełdowi oraz lifestylowi, a także szkoleniowcy z wieloletnim doświadczeniem dzielą się swoją wiedzą nt. pracy z mediami.








.gif)

 |
| |
| |
|