Contract Clinical Research Organization Market Expected to Surpass USD 165.56 Billion by 2032, CAGR of 9.5%
Contract Clinical Research Organization Market Research Report Information By Service Type, By Therapeutic Area, By End User, and By Geography
KS, UNITED STATES, May 9, 2025 /EINPresswire.com/ -- Contract Clinical Research Organization Market OverviewThe Global Contract Clinical Research Organization (CRO) Market is poised for substantial growth in the coming years, with its value expected to rise from USD 87.71 billion in 2025 to USD 165.56 billion by 2032, growing at a compound annual growth rate (CAGR) of 9.5% from 2025 to 2032. This growth is driven by increasing outsourcing of clinical trials by pharmaceutical and biotechnology companies, a surge in drug development activities, and a growing focus on cost efficiency and operational flexibility.
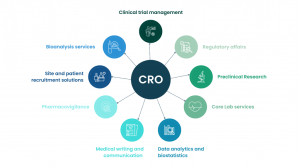
Contract Clinical Research Organizations (CROs) are entities that provide support to the pharmaceutical, biotechnology, and medical device sectors in the form of outsourced research services. These services range from clinical trial management to data management, regulatory affairs, medical writing, and pharmacovigilance. As the complexity of clinical trials continues to rise, and as global regulatory landscapes evolve, sponsors are increasingly turning to CROs to navigate these challenges efficiently and cost-effectively. This trend is accelerating the growth of the CRO market on a global scale.
Key Market Drivers
Rising R&D Expenditure in the Pharmaceutical Industry: With drug development costs reaching record highs, pharmaceutical and biotech companies are outsourcing clinical research activities to CROs to reduce costs and focus on core competencies.
Surge in Clinical Trials for Chronic and Rare Diseases: Increasing incidence of chronic conditions such as cancer, cardiovascular diseases, and rare genetic disorders is fueling the demand for extensive clinical research and trial services.
Expansion of Biologics and Biosimilars Pipeline: The rising focus on biologics and biosimilars, which often require complex clinical studies, is boosting demand for experienced CROs with specialized capabilities.
Technological Advancements in Clinical Trials: The adoption of decentralized clinical trials, wearable technology, real-time data analytics, and AI-driven trial management tools is transforming the CRO landscape and enhancing trial efficiency.
Globalization of Clinical Trials: Clinical trials are increasingly being conducted in emerging markets due to cost advantages, diverse patient populations, and favorable regulatory environments, thereby increasing demand for CROs with global operational footprints.
Request Your Sample Copy of the US Tariff Impact Analysis Now –https://www.coherentmarketinsights.com/insight/request-sample/7680
Competitive Landscape
The global CRO market is moderately consolidated, with several key players operating globally and many regional firms providing niche or specialized services. Major players include:
IQVIA
Labcorp Drug Development
Parexel International Corporation
Syneos Health
PPD (Thermo Fisher Scientific)
ICON plc
Medpace Holdings, Inc.
Charles River Laboratories
Wuxi AppTec
Pharmaceutical Product Development (PPD)
These companies are investing in technological innovation, expanding their global footprints, and acquiring or partnering with local CROs to enhance their capabilities and reach.
Market Segmentation
By Service Type:
Clinical Trial Services (Phase I-IV)
Regulatory Affairs
Data Management Services
Laboratory Services
Medical Writing
Pharmacovigilance
Others (site management, logistics, etc.)
By Therapeutic Area:
Oncology
Cardiovascular
Neurology
Infectious Diseases
Metabolic Disorders
Others
By End-User:
Pharmaceutical Companies
Biotechnology Companies
Medical Device Companies
Academic & Research Institutions
Regional Insights
North America dominates the global CRO market, attributed to strong pharmaceutical infrastructure, large R&D budgets, and the presence of major CROs.
Europe follows closely, with a mature healthcare research environment and supportive regulatory frameworks.
Asia-Pacific is projected to be the fastest-growing region, owing to the increasing number of clinical trials in countries like China, India, South Korea, and Japan. Cost-efficiency and access to large patient populations make this region particularly attractive.
Latin America, the Middle East, and Africa are emerging markets showing increasing interest in clinical research investments and CRO partnerships.
Get Up to 25% Discount on the US Tariff Impact Analysis Report –https://www.coherentmarketinsights.com/insight/buy-now/7680
Challenges and Opportunities
Challenges:
Complex regulatory requirements across regions
High competition and pricing pressures
Ensuring data integrity and patient recruitment in decentralized trials
Opportunities:
Increasing demand for virtual and hybrid clinical trials
Growing need for niche CROs in rare disease and personalized medicine research
Expanding CRO support in early-stage drug discovery
Future Outlook
The Global Contract CRO Market is expected to continue its upward trajectory, driven by innovation in trial methods, globalization of research, and the continuous pursuit of efficiency by pharmaceutical and biotech sponsors. As clinical trials become more complex and decentralized, CROs that offer comprehensive, tech-enabled, and adaptive services will lead the market.
✅ Key Benefits:
✦ Quantitative analysis of market segments, trends, estimations, and dynamics (2025-2032).
✦ Insights into key drivers, restraints, and opportunities.
✦ Porter's Five Forces analysis for strategic decision-making.
✦ Segmentation analysis to identify market opportunities.
✦ Revenue mapping of major countries by region.
✦ Benchmarking and positioning of market players.
✦ Analysis of regional and global trends, key players, and growth strategies.
Why You Should Buy This Report:
■ The impact of technological advancements and emerging industry trends
■ Regulatory and policy shifts and their implications for stakeholders
■ Competitive landscape analysis, including key player profiles and growth strategies
■ Major market challenges like supply chain issues and evolving consumer behavior
■ Opportunities in new products, applications, and potential investment areas
This report delivers actionable insights via secondary research, direct stakeholder interviews, and expert validation through Coherent Market Insights' extensive regional database.
What Happens When Strangers from 96 Countries Share a Home in Japan
Vetcon HVAC & Plumbing Services of Orlando Opens New Location to Serve Central Florida with Expert Commercial Services
XENOptics Wins Two Awards for Smart Optical Switches at Interop Tokyo 2025
Kalendarium
Więcej ważnych informacji
 Jedynka Newserii
Jedynka Newserii

 Jedynka Newserii
Jedynka Newserii

Prawo

Przedsiębiorcy chcą uproszczenia kontroli celno-skarbowych i podatkowych. Wskazują na potrzebę dialogu z kontrolerami
Poprawa relacji z fiskusem i urzędnikami przeprowadzającymi kontrole to jeden z najczęściej podnoszonych postulatów przez przedsiębiorców. W ramach deregulacji prowadzonej przez rząd domagają się oni większej współpracy i otwartości na wyjaśnienia ze strony organów podatkowych, jasnej interpretacji przepisów, partnerskiego traktowania oraz skrócenia procesu przedawnienia. Te zmiany mogłyby poprawić atmosferę i klimat dyskusji między przedsiębiorcami a rządem.
Bankowość
Banki zainteresowane projektami gospodarki obiegu zamkniętego. Chętniej finansują takie inwestycje

Rynek zrównoważonego finansowania rośnie. Banki stawiają na rozwój oferty zielonych instrumentów, które przedsiębiorcy i inne instytucje mogą przeznaczyć na sfinansowanie inwestycji z różnych obszarów ESG. Są wśród nich zarówno zielone obligacje, jak i kredyty połączone z realizacją konkretnych celów klimatycznych. Jednym z obszarów, które chcą finansować firmy w ramach ESG, jest gospodarka obiegu zamkniętego, czyli zamykanie obiegu produktów w myśl zasady reduce, reuse i recycle (ogranicz, użyj ponownie, przetwórz).
Handel
E-papierosy i aromatyzowane saszetki nikotynowe mogą zniknąć z rynku. Ministerstwo Zdrowia chce całkowitego zakazu ich sprzedaży

W wykazie prac legislacyjnych pojawiła się nowa propozycja Ministerstwa Zdrowia, która przewiduje zakaz wprowadzania do obrotu papierosów elektronicznych jednorazowego użytku – zarówno tych z nikotyną, jak i bez niej, oraz zakaz stosowania aromatów w woreczkach nikotynowych. Projekt trafił do konsultacji społecznych. To już kolejna regulacja sektora tytoniowego w ostatnich miesiącach. Przedstawiciele biznesu podkreślają, że to chaos regulacyjny, który wpływa na brak poczucia pewności prawnej i decyzje inwestycyjne.
Partner serwisu
Szkolenia

Akademia Newserii
Akademia Newserii to projekt, w ramach którego najlepsi polscy dziennikarze biznesowi, giełdowi oraz lifestylowi, a także szkoleniowcy z wieloletnim doświadczeniem dzielą się swoją wiedzą nt. pracy z mediami.







.gif)

 |
| |
| |
|