IPQS Launches Revolutionary Email Verification Database to Strengthen Fraud Prevention
Introducing a new product to combat fraud by detecting risky email credentials–while eliminating latency and enhancing data residency compliance.
LAS VEGAS, NV, UNITED STATES, May 15, 2025 /EINPresswire.com/ -- IPQS, a global leader in fraud prevention and risk intelligence, is proud to announce the launch of its IPQS Email Verification Database. This database is the first of its kind, enabling businesses to validate email addresses at scale. It reduces the need for external API calls for every fraud check, and makes it easier to comply with data privacy regulations.
The IPQS Email Verification Database enables businesses to identify fraudulent, disposable, or suspicious emails with unparalleled accuracy by tapping into IPQS’s vast repository of email reputation data. By analyzing factors such as email age, domain reputation, and historical fraud associations, companies can significantly enhance fraud detection while improving customer trust. Additionally, businesses can maintain better email hygiene by filtering out invalid or risky email addresses, improving deliverability rates and sender reputation.
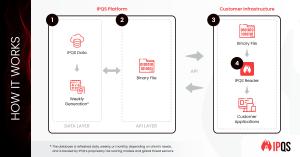
Email validation is essential for multi-layered fraud detection. However, companies may be wary of adding latency from additional risk checks or face regulatory restrictions on sharing customer data. Now, businesses can leverage email risk data in a locally hosted file. This data is delivered in a binary format which ensures fast downloads, alongside a reader that supports seamless integration into your applications and across all major programming languages.
The Next Generation of Email Intelligence
With billions of emails in its database, and growing daily, IPQS provides businesses with the most comprehensive access to granular email risk intelligence. This enables organizations to detect high-risk users, block fraudulent account registrations, and prevent payment fraud at scale. Delivered securely via an API, the database is updated on a daily, weekly, or monthly basis, depending on business requirements.
On-Premise Deployment: Eliminates API latency by processing email validation and fraud detection directly within company infrastructure.
Lightweight Design: Delivered in a proprietary binary format with a reader, enabling fast downloads and seamless integration.
Regulatory Compliance: Supports data residency regulatory requirements for companies that cannot share customer data with third-party providers.
Unmatched Data Accuracy: IPQS has been building its proprietary datasets for over a decade, providing industry-leading accuracy in detecting compromised and high-risk email addresses.
Email List Hygiene: Improves deliverability rates for email communication by helping to maintain a clean, verified contact list.
A Revolution in Fraud Prevention
Regarding this innovation’s impact, IPQS CEO Dennis Weiss said: “Fraud prevention is only as good as the data behind it. With the IPQS Email Verification Database, businesses can tap into the freshest, most comprehensive email risk intelligence—processed instantly, on their own infrastructure. This launch sets a new standard for speed, accuracy, and compliance in fraud detection.”
The IPQS Email Verification Database is available now and integrates seamlessly with IPQS’s full suite of fraud prevention tools, including IP address, device, phone, and cyber risk assessments. Businesses can schedule a demo or learn more by visiting www.ipqs.com/demo.
Lizzie Clitheroe
IPQS
+1 800-713-2618
lizzie|ipqs.com| |lizzie|ipqs.com
Visit us on social media:
LinkedIn
Instagram
Facebook
YouTube
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
DXRacer, 전설적인 저격수 kennyS와 손잡고 e스포츠 역사의 새로운 장을 열다
NEXCOM EBC 370 Powers Autonomous Robotics for Industrial Applications
The End Brain Cancer Initiative Partners with Curis to Pre-qualify for PCNSL TakeAim Lymphoma Clinical Trial
Kalendarium
Więcej ważnych informacji
 Jedynka Newserii
Jedynka Newserii

 Jedynka Newserii
Jedynka Newserii

Ochrona środowiska

Z powodu braku przejrzystego prawa branża recyklingu odkłada inwestycje. Firmy apelują o szybkie wdrożenie przepisów
Branża recyklingu stoi przed wyzwaniami związanymi z wdrożeniem systemu kaucyjnego, systemu rozszerzonej odpowiedzialności producenta i rozporządzenia PPWR. Brakuje jednak odpowiednich przepisów dostosowujących polskie prawo i realia do unijnych regulacji. W efekcie utrzymującej się niepewności prawnej między 2018 a 2023 rokiem co trzeci zakład recyklingu zamknął działalność. Wiele firm odkłada inwestycje, czekając na uregulowanie rynku. Podobna niepewność dotyczy też producentów opakowań.
Transport
Testowanie pojazdów zautomatyzowanych wkrótce będzie możliwe. To odpowiedź na postulaty przedsiębiorców

Kończą się prace nad przepisami, które mają usprawnić prace badawcze nad pojazdami zautomatyzowanymi. Ma to być odpowiedź na postulaty przedsiębiorców, którzy wskazywali na potrzebę pilnej zmiany przepisów w zakresie testowania pojazdów autonomicznych. Obecne regulacje nie sprzyjają postępowi technologicznemu i rozwoju autonomiczności pojazdów, o czym świadczy bardzo niewielka liczba wydanych uprawnień do ich prowadzenia.
Firma
Przedsiębiorcom coraz bardziej doskwiera niestabilność i skomplikowanie przepisów podatkowych. Problemem są też niejasne ich interpretacje

Polscy przedsiębiorcy często negatywnie oceniają jakość przepisów podatkowych – wynika z raportu „Przedsiębiorcy pod lupą fiskusa 2025” przygotowanego przez firmę doradztwa podatkowego MDDP we współpracy z Konfederacją Lewiatan. Ich niepokój budzą niejednolite interpretacje przepisów i niepewność prawa podatkowego, które cały czas jest modyfikowane. Wśród kluczowych zmian w obszarze podatków, które będą dotyczyć praktycznie wszystkich przedsiębiorców, są m.in. wprowadzenie Krajowego Systemu e-Faktur czy zmiany w podatku od nieruchomości.
Partner serwisu
Szkolenia

Akademia Newserii
Akademia Newserii to projekt, w ramach którego najlepsi polscy dziennikarze biznesowi, giełdowi oraz lifestylowi, a także szkoleniowcy z wieloletnim doświadczeniem dzielą się swoją wiedzą nt. pracy z mediami.










.gif)

 |
| |
| |
|