Qalitex Laboratories Shares Guidance on Navigating Amazon’s Evolving Compliance Requirements
Qalitex outlines key steps for Amazon sellers to stay compliant with evolving policies, focusing on documentation, labeling, and regulatory alignment.
“Amazon’s compliance expectations change constantly,” said Nour Abochama, Vice President for Operations at Qalitex. “We’ve worked with brands that did everything right at launch, only to be flagged later because the platform updated its policies. Our role is to help brands prepare for what’s next—so they’re not caught off guard.”
Amazon’s Increasing Oversight in Regulated Product Categories
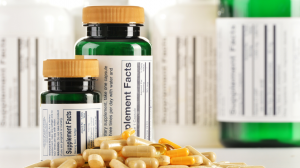
Amazon has intensified its focus on certain product categories, particularly those that relate to health, wellness, and personal care. This includes vitamins, herbal supplements, skincare products, ingestibles, and cosmetics—categories that are often subject to scrutiny from the FDA, FTC, and other regulatory bodies in addition to Amazon’s own internal policies.
Over the past several years, Amazon has implemented stricter enforcement mechanisms such as:
Requiring batch-specific Certificates of Analysis (COAs)
Requesting evidence to support health or performance claims
Automatically delisting products with conflicting or outdated documentation
Increasing reliance on automated systems to detect noncompliance
This evolution has created a landscape where even small discrepancies—such as a mismatched product label or ambiguous claim—can result in enforcement action.
“It’s not just about whether your product is safe,” Abochama explained. “It’s about whether your entire listing—from label copy to lab results—meets a constantly evolving set of expectations.”
Common Issues That Trigger Product Takedowns
Qalitex has identified recurring issues that sellers face, often unknowingly, that contribute to listings being flagged or removed. These include:
1. Claims That Lack Sufficient Documentation
Terms such as “clinically proven,” “boosts immunity,” or “reduces inflammation” require substantiation. Without robust scientific evidence or clinical data on file, these claims are considered noncompliant.
2. Expired or Missing COAs
Many sellers are unaware that Amazon requires up-to-date documentation for every batch. Submitting a COA from a previous manufacturing run—or failing to submit one altogether—can result in rejection.
3. Inconsistencies Between Packaging and Listings
Discrepancies between what a product label states and what is written on the Amazon listing (title, bullets, A+ content) can result in listings being flagged for misleading information.
4. Non-standardized Documentation
Even when brands have proper testing, if the formatting doesn’t meet Amazon’s technical requirements, submissions can be denied or delayed.
The Case for Preventive Compliance Practices
Rather than reacting to violations after a product is taken down, Qalitex emphasizes a preventive approach. Their team encourages brands to treat compliance as an integral part of operational planning, rather than a final checkbox before launch.
“Our work often starts before a product is even listed,” Abochama noted. “We work with clients to assess product risk, review all relevant documents, and ensure consistency across platforms. The goal is to prevent violations—not just resolve them.”
Qalitex’s typical client workflow includes:
Initial compliance review of product labeling, ingredients, and listing content
Coordination with certified laboratories to generate Amazon-acceptable test results
Formatting and submitting required documentation in alignment with Amazon’s preferred structures
Flagging potential issues in product claims, descriptions, or packaging before publication
Monitoring Evolving Policy and Regulatory Updates
Compliance isn’t static. Amazon regularly updates its Seller Central policies, and regulators like the FDA and FTC publish new guidance that may affect product positioning, testing thresholds, or labeling language.
Qalitex Laboratories monitors both Amazon and federal regulatory channels to keep clients informed about shifts that may affect their listings. When relevant changes occur—such as ingredient restrictions or new documentation requirements—the team alerts clients and helps them adjust preemptively.
“It’s important to stay ahead of the updates,” Abochama said. “We don’t wait for clients to come to us with a problem. We aim to catch the change before it causes disruption.”
Why Compliance Has Become a Competitive Consideration
Qalitex emphasizes that robust compliance is increasingly tied to a brand’s long-term viability on Amazon. Delistings not only result in temporary loss of revenue, but they also impact visibility, customer trust, and product rankings.
Sellers with complete documentation and aligned listings are also more likely to pass periodic Amazon audits, which can occur randomly or in response to customer complaints. In high-growth categories, avoiding even a few days of downtime can significantly impact a brand’s performance metrics.
“Brands that invest in compliance early are often the ones that sustain long-term momentum,” Abochama said. “They can scale more confidently because their operations are already built to withstand scrutiny.”
No One-Size-Fits-All Approach
Qalitex works with both new and established brands and emphasizes that each product requires a different level of attention depending on category, formulation, and intended claims. Some sellers may only need documentation review, while others benefit from full-scale support across testing, auditing, and strategy.
“There’s no universal solution,” Abochama added. “Each client has a different risk profile. We listen closely to their goals, then recommend what’s necessary to meet both Amazon’s standards and their own.”
Anticipating Continued Policy Developments
Looking forward, Qalitex expects Amazon to continue tightening enforcement across consumer health categories. This may include additional ingredient disclosures, expanded documentation requirements, and shorter COA expiration windows.
The company also notes that international expansion adds additional layers of regulatory responsibility, especially for brands entering markets in Canada, the EU, or the UK.
“What we’re seeing now is just the start,” said Abochama. “The compliance landscape will continue to grow in complexity. Being proactive now means fewer disruptions—and better readiness—later.”
About Qalitex Laboratories
Qalitex Laboratories is an Irvine, California-based compliance support provider for consumer brands in supplements, cosmetics, and wellness. The company helps businesses navigate Amazon’s documentation requirements through regulatory guidance, laboratory coordination, and listing audits. Qalitex works with clients seeking a structured, compliant approach to marketplace readiness and brand integrity.
Nour Abochama
Qalitex
email us here
Visit us on social media:
LinkedIn
Instagram
Facebook
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
AllScale Partners with BNB Chain to Power Global Stablecoin Payments for Micro Businesses
AllScale joins Amber.ac's BUILD_QUESTS 2025 as an Official Partner to Support Stablecoin Innovation
Michelin-Trained Chef Reimagines Intimate Luxury Dining
Więcej ważnych informacji
 Jedynka Newserii
Jedynka Newserii

 Jedynka Newserii
Jedynka Newserii

Handel

Mercosur to tylko wierzchołek góry lodowej. UE ma ponad 40 umów handlowych, które mogą destabilizować rynek rolny
Umowa handlowa między UE a krajami Mercosur może znacząco zaburzyć konkurencję na rynku rolnym i osłabić pozycję unijnych, w tym polskich, producentów – ostrzegają rolnicy i producenci żywności. Umowie sprzeciwia się część krajów unijnych, które domagają się klauzuli ochronnych oraz limitów importowych. – Problemem jest jednak nie tylko ta konkretna umowa. Chodzi o cały system wolnego handlu, który się kumuluje z dziesiątek innych porozumień – podkreśla Andrzej Gantner, wiceprezes Polskiej Federacji Producentów Żywności.
Firma
Dzięki zdalnej weryfikacji tożsamości z wykorzystaniem AI firmy zminimalizowały liczbę oszustw. Rozwiązania wykorzystuje głównie sektor finansowy

Z najnowszych danych Eurostatu wynika, że w 2024 roku 5,9 proc. polskich firm korzystało z rozwiązań z zakresu sztucznej inteligencji. W 2023 roku był to odsetek na poziomie 3,67 proc. Wciąż jednak jest to wynik poniżej średniej unijnej, która wyniosła 13,48 proc. Jednym z obszarów, który cieszy się coraz większym zainteresowaniem wśród przedsiębiorców, jest weryfikacja tożsamości przez AI, zwłaszcza w takich branżach jak bankowość, ubezpieczenia czy turystyka. Jej zastosowanie ma na celu głównie przeciwdziałać oszustwom i spełniać wymogi regulacyjne.
Prawo
Daniel Obajtek: Własne wydobycie i operacyjne magazyny to filary bezpieczeństwa. Zgoda na magazyny gazu poza krajem to rezygnacja z suwerenności energetycznej

Były prezes Orlenu ostrzega przed zmianami w ustawie o zapasach ropy naftowej, produktów naftowych i gazu ziemnego. Jego zdaniem przygotowana przez rząd nowelizacja tzw. ustawy magazynowej i ujednolicanie unijnej polityki energetycznej to zagrożenie dla bezpieczeństwa energetycznego Polski. W jego opinii tylko silna spółka narodowa, własne wydobycie, krajowe magazyny i zbilansowany miks energetyczny zapewnią Polsce bezpieczeństwo i konkurencyjność.
Partner serwisu
Szkolenia

Akademia Newserii
Akademia Newserii to projekt, w ramach którego najlepsi polscy dziennikarze biznesowi, giełdowi oraz lifestylowi, a także szkoleniowcy z wieloletnim doświadczeniem dzielą się swoją wiedzą nt. pracy z mediami.





![Nestlé w Polsce podsumowuje wpływ na krajową gospodarkę. Firma wygenerowała 0,6 proc. polskiego PKB [DEPESZA]](https://www.newseria.pl/files/1097841585/fabryka-nesquik_1,w_85,r_png,_small.png)



.gif)

 |
| |
| |
|