Active Pharmaceutical Ingredients (API) Market Forecast to Cross US$ 466.9 Bn by 2035 – Transparency Market Research
Active Pharmaceutical Ingredients market is poised for significant growth, expanding biopharmaceutical production, and increasing outsourcing of manufacturing.
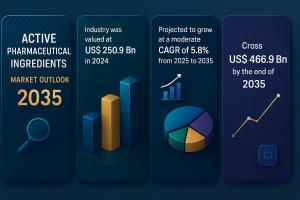
WILMINGTON, DE, UNITED STATES, August 15, 2025 /EINPresswire.com/ -- The global Active Pharmaceutical Ingredients (API) market was valued at USD 250.9 Billion in 2024 and is projected to cross USD 466.9 Billion by 2035, expanding at a CAGR of 5.8% from 2025 to 2035. Growth is driven by rising demand for generic medicines, increasing R&D investments, expanding pharmaceutical manufacturing capabilities in emerging economies, and the growing burden of chronic and infectious diseases worldwide.Don’t miss out on the latest market intelligence. Get your sample report copy today - https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=1690
Market Introduction
Active Pharmaceutical Ingredients (APIs) are the biologically active components in drugs that produce the intended therapeutic effect. APIs are combined with excipients—inactive substances—to form the final pharmaceutical product. They are crucial for both branded and generic drugs, spanning therapeutic categories such as cardiovascular, anti-infective, oncology, neurological, and more. APIs can be produced through synthetic or natural processes and are manufactured either in-house (captive production) or through outsourcing to contract manufacturing organizations (CMOs).
Analyst Viewpoints
The API market is transitioning toward more resilient, technologically advanced, and geographically diversified supply chains. With the increasing penetration of biosimilars, biologics, and high-potent APIs, manufacturers are focusing on both innovation and cost efficiency.
Three notable shifts shaping the industry include:
Surge in Generic Drug Demand – Patent expiries for blockbuster drugs are accelerating generic API production.
Advanced Manufacturing Adoption – Continuous manufacturing, greener chemistry, and automation are improving scalability and sustainability.
Regional Supply Chain Strengthening – Governments and companies in Asia-Pacific, North America, and Europe are investing in local API production to reduce import dependency.
Analysis of Key Players in the Active Pharmaceutical Ingredients (API) Market
Leading companies are expanding production capacity, investing in R&D, and pursuing strategic collaborations to strengthen API supply chains and meet global demand. Prominent players include:
Teva Pharmaceutical Industries Ltd.
Pfizer Inc.
Mangalam Drugs & Organics Limited
Viatris Inc.
Lonza
Piramal Pharma Solutions
HISUN USA, Inc.
Ipca Laboratories Ltd.
AbbVie Inc.
Alembic Pharmaceuticals Limited
Biocon
Merck KGaA
Boehringer Ingelheim
Cambrex Corporation
Dr. Reddy’s Laboratories Ltd.
Sun Pharmaceutical Industries Ltd.
Cipla
Key Developments
September 2024 – Wanbury launched a new API product portfolio covering antidepressants, anti-diabetics, analgesics, anti-inflammatory drugs, anti-histamines, antitussives, and anesthetics.
March 2024 – Noramco introduced the Noramco Group, integrating Purisys, Noramco, and Halo Pharma to form a robust North American API and drug product supply chain solution.
Key Growth Drivers
Rising Demand for Generic Drugs – Patent expiries and cost pressures drive API demand for generic formulations.
Increasing R&D Investment – Supports innovation in biologics, biosimilars, and specialty APIs.
Expanding Pharmaceutical Manufacturing in Emerging Markets – Particularly in Asia-Pacific and Latin America.
Advancements in Synthetic API Production – Ensures consistent quality, scalability, and supply stability.
Government Support for Local API Production – Incentives to reduce import dependence.
Opportunities
Expansion of precision and personalized medicine APIs.
Growth in sustainable and green chemistry production processes.
Rising outsourcing demand to contract manufacturing organizations.
Increasing use of high-potent APIs (HPAPIs) in oncology and specialty drugs.
Challenges
High manufacturing and compliance costs (EU-GMP, FDA).
Complex production processes and narrow profit margins.
Regulatory hurdles and lengthy approval timelines.
Skilled workforce shortages in API manufacturing.
Market Segmentation
By Molecule Type:
Acetaminophen • Naproxen • Furosemide • Nitrofurantoin • Sulfadoxine • Pyrimethamine • Amodiaquine • Atazanavir Sulfate • Nimesulide • Ciprofloxacin • Piperaquine Phosphate Sotalol • Levetiracetam • Diclofenac • Azithromycin • Others
By Production Type:
Captive/In House • Outsourcing
By Product Type:
Low Potent API • High Potent API
By API Type:
Synthetic • Natural
By Scale:
Pilot • Large
By Application:
Commercial • Research
By End User:
Pharmaceutical Companies • Biotechnological Companies • CMOs • Others
By Region:
North America • Europe • Asia-Pacific • Latin America • Middle East & Africa
Regional Outlook
North America remains the leading region due to its advanced pharmaceutical manufacturing base, strong R&D ecosystem, and high incidence of chronic diseases requiring both generic and branded drugs. The U.S. dominates the region, driven by domestic production incentives, established CMOs, and a highly regulated yet supportive market environment. Asia-Pacific is expected to witness the fastest growth due to government-led initiatives for API self-reliance, lower production costs, and expanding local pharma industries in India and China.
Future Prospectus
By 2035, the API market will be characterized by digitally integrated manufacturing, AI-driven quality control, sustainable chemical synthesis, and localized supply chains. High-potent and specialty APIs will see the strongest growth, especially in oncology and personalized therapies. Governments will continue incentivizing domestic API production to safeguard public health security.
What Is in This Report?
Global & regional market size forecasts (2025–2035)
Detailed segmentation by molecule type, production type, product type, API type, scale, application, and end user
Market drivers, restraints, opportunities, and emerging trends
Competitive landscape & key player strategies
Regulatory and manufacturing compliance analysis
Porter's Five Forces & value chain analysis
Why Buy This Report?
Strategic Planning – Leverage data-backed forecasts for investment decisions.
Innovation Insights – Track emerging API technologies and manufacturing trends.
Competitive Benchmarking – Compare top API producers globally.
Risk Management – Understand regulatory, supply chain, and operational risks.
Growth Targeting – Identify high-potential segments and regions.
Conclusion
The Active Pharmaceutical Ingredients (API) market is evolving into a more resilient, technology-driven, and sustainable industry. Companies that invest in high-potent API capabilities, adopt greener manufacturing processes, and develop strong regional supply networks will lead the global market in the coming decade.
More Trending Related Research Reports –
Biotech API Manufacturing Services Market - https://www.transparencymarketresearch.com/biotech-api-manufacturing-services.html
Peptide Therapeutics Contract API Manufacturing Market - https://www.transparencymarketresearch.com/peptide-therapeutics-contract-api-manufacturing-market.html
Pharmaceutical Intermediates Market - https://www.transparencymarketresearch.com/pharmaceutical-intermediates-market.html
Naproxen Market - https://www.transparencymarketresearch.com/naproxen-market.html
Oncology Small Molecule Drugs Market - https://www.transparencymarketresearch.com/oncology-small-molecule-drugs-market.html
Bioanalytical Testing Services Market - https://www.transparencymarketresearch.com/bioanalytical-testing-services-market.html
Drug Delivery Systems Market - https://www.transparencymarketresearch.com/drug-delivery-systems-market.html
Post-operative Pain Therapeutics Market - https://www.transparencymarketresearch.com/postoperative-pain-therapeutics-market.html
Acute Viral Rhinosinusitis Treatment Market - https://www.transparencymarketresearch.com/acute-viral-rhinosinusitis-treatment-market.html
Medical and Diagnostic Laboratory Services Market - https://www.transparencymarketresearch.com/medical-diagnostic-laboratory-services-market.html
PAP and Paracetamol Market - https://www.transparencymarketresearch.com/pap-paracetamol-market.html
Amlodipine Market - https://www.transparencymarketresearch.com/amlodipine-market.html
Budesonide Inhaler Market - https://www.transparencymarketresearch.com/budesonide-inhaler-market.html
Pharmaceutical Continuous Manufacturing Market - https://www.transparencymarketresearch.com/pharmaceutical-continuous-manufacturing-technology-market.html
Metformin Hydrochloride API Market - https://www.transparencymarketresearch.com/metformin-hydrochloride-api-market.html
Pharmaceutical Products and CMO Market - https://www.transparencymarketresearch.com/latin-america-pharmaceutical-products-cmo-market.html
Pharmaceutical Testing and Analytical Services Market - https://www.transparencymarketresearch.com/pharmaceutical-testing-analytical-services-market.html
High Potency Active Pharmaceutical Ingredients (HPAPI) Market - https://www.transparencymarketresearch.com/high-potency-active-pharmaceutical-Ingredients-market.html
About Us Transparency Market Research -
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. The firm scrutinizes factors shaping the dynamics of demand in various markets. The insights and perspectives on the markets evaluate opportunities in various segments. The opportunities in the segments based on source, application, demographics, sales channel, and end-use are analysed, which will determine growth in the markets over the next decade.
Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision-makers, made possible by experienced teams of Analysts, Researchers, and Consultants. The proprietary data sources and various tools & techniques we use always reflect the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in all of its business reports.
Contact Us -
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Blog: https://tmrblog.com
Email: sales@transparencymarketresearch.com
Atil Chaudhari
Transparency Market Research Inc.
+1 518-618-1030
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Rony Jabour Leads Workplace Emergency Preparedness Keynote in Boston During Safe + Sound Week
Nu Dai Wellness Opens Premier Addiction Treatment Center in Orange County
PARK WEBCAMS SHOW BLUE SKIES, 100 MILE VIEWS AT GRAND CANYON SOUTH RIM
Więcej ważnych informacji
 Jedynka Newserii
Jedynka Newserii

 Jedynka Newserii
Jedynka Newserii

Konsument
Polacy nie korzystają z hossy trwającej na warszawskiej giełdzie. Na wzrostach zarabiają głównie inwestorzy zagraniczni
Od października 2022 roku na rynkach akcji trwa hossa, nie omija ona także warszawskiej giełdy. Mimo to inwestorzy indywidualni odpowiadają zaledwie za kilkanaście procent inwestycji, a o wzrostach decyduje i na nich zarabia głównie kapitał z zagranicy. Widać to również po napływach i odpływach do i z funduszy inwestycyjnych. Zdaniem Tomasza Koraba, prezesa EQUES Investment TFI, do przekonania Polaków do inwestowania na rodzimej giełdzie potrzeba zysków z akcji, informacji o tych zyskach docierającej do konsumentów oraz czasu.
Polityka
Obowiązek zapełniania magazynów gazu w UE przed sezonem zimowym ma zapewnić bezpieczeństwo dostaw. Wpłynie też na stabilizację cen

Unia Europejska przedłuży przepisy z 2022 roku dotyczące magazynowania gazu. Będą one obowiązywać do końca 2027 roku. Zobowiązują one państwa członkowskie do osiągnięcia określonego poziomu zapełnienia magazynów gazu przed sezonem zimowym. Magazyny gazu pokrywają 30 proc. zapotrzebowania Unii Europejskiej na niego w miesiącach zimowych. Nowe unijne przepisy mają zapewnić stabilne i przystępne cenowo dostawy.
Infrastruktura
Gminy zwlekają z uchwaleniem planów ogólnych zagospodarowania przestrzennego. Może to spowodować przesunięcie terminu ich wejścia w życie

Reforma systemu planowania i zagospodarowania przestrzennego rozpoczęła się we wrześniu 2023 roku wraz z wejściem w życie większości przepisów nowelizacji ustawy z 27 marca 2003 roku. Uwzględniono w niej plany ogólne gminy (POG) – nowe dokumenty planistyczne, za których przygotowanie mają odpowiadać samorządy. Rada Ministrów w kwietniu br. uchwaliła jednak ustawę o zmianie ustawy z 7 lipca 2023 roku, a jej celem jest zmiana terminu obowiązywania studiów uwarunkowań i kierunków zagospodarowania przestrzennego gmin na 30 czerwca 2026 roku. Wskazana data może nie być ostateczna z uwagi na to, że żadna z gmin nie uchwaliła jeszcze POG.
Partner serwisu
Szkolenia

Akademia Newserii
Akademia Newserii to projekt, w ramach którego najlepsi polscy dziennikarze biznesowi, giełdowi oraz lifestylowi, a także szkoleniowcy z wieloletnim doświadczeniem dzielą się swoją wiedzą nt. pracy z mediami.








.gif)

 |
| |
| |
|