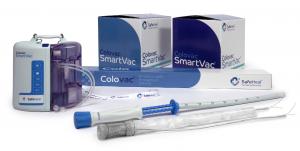
SafeHeal® Receives European Marketing Approval Under MDR for Colovac® Anastomosis Protection Technology
Marketing approval allows imminent commercialization of the Colovac device in key EU markets
NEW YORK, NY, UNITED STATES, August 14, 2025 /EINPresswire.com/ -- SafeHeal®, a leading innovator in the field of colorectal cancer surgery, today announced that it has been granted European Union marketing approval for its Colovac device under the new Medical Device Regulation (EU MDR 2017/745, Medical Devices, Annex IX Chapter I). This significant milestone confirms the company’s compliance with the EU’s rigorous safety and performance standards, enabling commercial distribution of Colovac across the European Union. Colovac is intended as an alternative to temporary diverting ostomy for patients undergoing colorectal cancer resection. 1,2“This is a pivotal achievement for our company and a testament to the dedication of our regulatory, clinical, and engineering teams,” said Chris Richardson, President and Chief Executive Officer of SafeHeal. “We are now ready to bring the clinical and economic benefits of Colovac to healthcare providers and patients throughout Europe.”
The Colovac endoluminal bypass system is a less-invasive alternative to temporary diverting ostomy, the current standard of care for patients undergoing colorectal resection. Diverting ostomy is applied prophylactically to most patients today undergoing a low anterior resection (LAR) and anastomosis. The ostomy temporarily diverts the stool away from the healing anastomosis to the outside of the body and into an ostomy bag. In most cases, the ostomy is needed only until the anastomosis has healed, and then it can be reversed, typically after 2-6 months. The eventual reversal of the ostomy requires another operation, with a second hospital stay, recovery period and associated complications. In some cases, the ostomy may not be reversed and becomes permanent. In addition to the potential surgical complications associated with ostomy procedures, patients may experience an impact to their quality of life due to social isolation, reduced physical activity and/or intimacy.
Colovac is an alternative to diverting ostomy, designed to eliminate the need for a temporary stoma in most patients. It aims to improve patient recovery and quality of life by eliminating stoma related complications including permanent stoma and eliminating the physical and emotional burden associated with stoma management and care.
“Navigating the MDR process is no small feat for any company, and gaining approval affirms the strength of our technology and the robust data supporting it. After conducting a thorough review of the data supporting the performance and safety of the device and SafeHeal’s quality management system, the EU Medical Device regulators wasted no time in recognizing the obvious clinical benefits Colovac provides to colorectal cancer patients,” said Richardson.
Colovac has been successfully studied in the U.S., Europe, and Asia and the U.S. Food and Drug Administration (FDA) has already granted the product Breakthrough Device Designation. Breakthrough Device designation is granted to novel products and allows FDA to expedite the review of innovative technologies that can improve the lives of people with life-threatening or irreversibly debilitating diseases or conditions.
1 Intended Purpose: The Colovac Anastomosis Protection Device is intended for use in patients requiring low anterior rectal anastomoses to limit stoma creation to only those patients requiring more time for anastomosis healing when the device is removed, allowing patients with a healed anastomosis to avoid stoma creation.
2 Indication for Use: The SafeHeal Colovac Device is indicated for use following open, laparoscopic, or robotic-assisted laparoscopic colorectal surgery in patients indicated for diverting ostomy.
ABOUT SAFEHEAL®
SafeHeal SAS, headquartered in Paris, France, and its wholly owned U.S. subsidiary, SafeHeal Inc., is a medical device company developing Colovac, a device intended as an alternative to diverting ostomy in patients undergoing colorectal surgery. Colovac is a flexible endoluminal bypass sheath designed to reduce the contact of fecal content at the anastomotic site following colorectal surgery. The device is placed endoluminally and is fully reversible. The device remains in place for approximately 10 days, until the body’s natural healing and tissue repair processes are complete, after which it is removed during an endoscopic procedure without the need for a second surgical intervention. This enables patients to resume their normal life without the stigma and complications associated with an ostomy procedure. In the U.S., Colovac is limited by Federal law to investigational use and not currently available for sale. For more information, please visit www.safeheal.com.
MEDIA CONTACTS
USA
Scott DePierro
Vice President U.S. Operations
and Global Business Development, SafeHeal
203-444-0279
sdepierro@safeheal.com
www.safeheal.com
Europe:
Karl-Heinz Blohm
Vice President, International, SafeHeal
+33 (0) 6 5181 7895
kblohm@safeheal.com
www.safeheal.com
Scott DePierro, VP US Operations & Global Business Dev
SafeHeal
+1 203-444-0279
sdepierro|safeheal.com| |sdepierro|safeheal.com
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
API Management Market to Hit USD 24.17 Billion By 2032, API Management Enhances Software Connectivity
2024 ThreeBestRated® Award-Winner Blake Goodman P.C., Attorney, Offers Essential Tips for Filing Chapter 7 Bankruptcy
Mauro Electric Inc. Expands into Full-Service HVAC, Offering Heating, Cooling, and Electrical Expertise All in One Place
Więcej ważnych informacji
 Jedynka Newserii
Jedynka Newserii

 Jedynka Newserii
Jedynka Newserii
Konsument
Polacy nie korzystają z hossy trwającej na warszawskiej giełdzie. Na wzrostach zarabiają głównie inwestorzy zagraniczni
Od października 2022 roku na rynkach akcji trwa hossa, nie omija ona także warszawskiej giełdy. Mimo to inwestorzy indywidualni odpowiadają zaledwie za kilkanaście procent inwestycji, a o wzrostach decyduje i na nich zarabia głównie kapitał z zagranicy. Widać to również po napływach i odpływach do i z funduszy inwestycyjnych. Zdaniem Tomasza Koraba, prezesa EQUES Investment TFI, do przekonania Polaków do inwestowania na rodzimej giełdzie potrzeba zysków z akcji, informacji o tych zyskach docierającej do konsumentów oraz czasu.
Polityka
Obowiązek zapełniania magazynów gazu w UE przed sezonem zimowym ma zapewnić bezpieczeństwo dostaw. Wpłynie też na stabilizację cen

Unia Europejska przedłuży przepisy z 2022 roku dotyczące magazynowania gazu. Będą one obowiązywać do końca 2027 roku. Zobowiązują one państwa członkowskie do osiągnięcia określonego poziomu zapełnienia magazynów gazu przed sezonem zimowym. Magazyny gazu pokrywają 30 proc. zapotrzebowania Unii Europejskiej na niego w miesiącach zimowych. Nowe unijne przepisy mają zapewnić stabilne i przystępne cenowo dostawy.
Infrastruktura
Gminy zwlekają z uchwaleniem planów ogólnych zagospodarowania przestrzennego. Może to spowodować przesunięcie terminu ich wejścia w życie

Reforma systemu planowania i zagospodarowania przestrzennego rozpoczęła się we wrześniu 2023 roku wraz z wejściem w życie większości przepisów nowelizacji ustawy z 27 marca 2003 roku. Uwzględniono w niej plany ogólne gminy (POG) – nowe dokumenty planistyczne, za których przygotowanie mają odpowiadać samorządy. Rada Ministrów w kwietniu br. uchwaliła jednak ustawę o zmianie ustawy z 7 lipca 2023 roku, a jej celem jest zmiana terminu obowiązywania studiów uwarunkowań i kierunków zagospodarowania przestrzennego gmin na 30 czerwca 2026 roku. Wskazana data może nie być ostateczna z uwagi na to, że żadna z gmin nie uchwaliła jeszcze POG.
Partner serwisu
Szkolenia

Akademia Newserii
Akademia Newserii to projekt, w ramach którego najlepsi polscy dziennikarze biznesowi, giełdowi oraz lifestylowi, a także szkoleniowcy z wieloletnim doświadczeniem dzielą się swoją wiedzą nt. pracy z mediami.









.gif)

 |
| |
| |
|