Cure-in enters global medical device market with ‘MR-SON,’ an MRI-guided automated breast biopsy robot
Targeting U.S. and European markets with precision and convenience-enhancing automation
PANGYO, GYEONGGI-DO, SOUTH KOREA, July 19, 2025 /EINPresswire.com/ -- Cure-in Inc. (co-CEOs Yung-Ho Jo and Samuel Byeongjun Park), a medical robotics company founded in 2020 by a research team from the Department of Biomedical Engineering at the National Cancer Center, aims to offer trust to medical professionals and comfort to patients. The company has developed and is commercializing an automated robotic system for confirming suspicious breast cancer lesions detected on MRI. The system is known as ‘MR-SON (MRI-guided Semi-Automated Needle Biopsy Robot System).’Development of MR-SON began in 2011. The robot can reduce procedure time by more than half compared to traditional manual methods. While manual procedures typically take around one hour, MR-SON can complete them in just over 30 minutes. After completing exploratory clinical trials, Cure-in is now preparing for pivotal domestic clinical trials and aims to become a leading global company in the field of image-guided robotic procedures.
The primary target customers for MR-SON are hospitals that utilize MRI equipment for breast cancer screening. Initially, the focus is on large cancer centers with a high volume of breast cancer patients and active use of MRI-guided biopsies. Once clinical stability is confirmed, expansion to mid-sized hospitals equipped with MRI will follow.
In regions like the United States, where travel between cities can be burdensome, patients at smaller hospitals often need to transfer to urban hospitals for biopsies after an MRI. With MR-SON, even hospitals lacking specialized staff can perform accurate and efficient biopsies, benefiting both hospitals and patients. Hospitals can retain patients, and patients can avoid long-distance travel and overnight stays, greatly improving convenience.
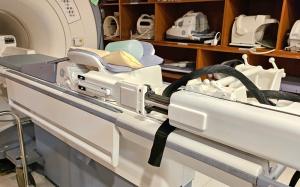
MRI is considered the most accurate imaging technology for breast cancer diagnosis, capable of detecting tumors invisible on ultrasound or X-rays. When suspicious areas are identified on an MRI, a follow-up MRI-guided biopsy is necessary for approximately 10-15% of all biopsy candidates. However, with conventional systems, the patient must be removed from the MRI machine, and medical staff must perform the procedure inside the MR suite. This process lengthens procedure time, increases patient discomfort, and raises the risk of failed procedures.
To overcome these limitations, Cure-in developed an automated breast biopsy system that combines an MR-compatible biopsy robot with image-guided navigation technology. The system features MR-safe needles and instruments, and is designed to be slim enough to approach from the side without altering existing breast coil structures. Thanks to its automated navigation feature, procedures can be performed without physically repositioning the patient, significantly reducing procedure time. As a result, the system is praised for improving accuracy while reducing patient discomfort.
Cure-in has designated the U.S. as its primary strategic market. With approximately 310,000 new breast cancer cases annually, high MRI adoption rates, and a strong receptiveness to advanced medical devices, the U.S. accounts for an estimated 50–60% of the MRI-guided biopsy market. Cure-in is optimistic about its prospects in the U.S. and plans to enter the European market next. In Europe, the company is targeting Germany first, given its large number of breast cancer patients and high MRI adoption, and will expand into other EU countries.
To enter global markets, Cure-in is seeking investment partners to establish local subsidiaries and commercialization infrastructure. It will also look for partners with nationwide medical device distribution and maintenance capabilities in the U.S.
Domestically, the company aims to initiate pivotal clinical trials by the end of 2025, complete them in the first half of 2026, and obtain regulatory approval in the second half, paving the way for sales to commence.
Cure-in recently relocated its base to Pangyo to enhance external communications and attract talent. CEO Samuel Byeongjun Park said, “We initially established the company in Goyang for close collaboration with the National Cancer Center, but Pangyo provides an optimal environment for meeting investors and partners and expanding our network.” He added, “We hope to grow alongside young and talented individuals in Pangyo.”
Cure-in is currently participating in the global acceleration program operated by the Techno Valley Planning Team of the Gyeonggi Business and Science Accelerator.
This program identifies promising startups aiming for global markets. It provides comprehensive support, ranging from office space to consulting for overseas market entry, IR pitching training, and participation in global demo days. Based at the Pangyo Startup Campus, the program is helping participating companies enhance their international expansion strategies and fundraising capabilities. Through tailored training and expert network connections, the program is recognized for laying the groundwork for global growth.
Pangyo Techno Valley is a global R&D hub that integrates Research (R), People (P), Information (I), and Trade (T) across the IT, BT, CT, NT, and mobility sectors. It is a leading innovation cluster in Gyeonggi-do, established to drive technological innovation, talent development, job creation, and international business competitiveness.
The Gyeonggi Business and Science Accelerator’s Techno Valley Innovation Headquarters has continuously promoted Pangyo Techno Valley’s value by hosting events such as the Pangyo Evening Meet-Up, Pan-Pan Day, and Pangyo Startup Investment Exchange In-Best Pangyo. These initiatives have facilitated networking between Pangyo companies, domestic and international investors, and the media. Similar events are planned for this year to support the growth and global expansion of Pangyo startups through various assistance programs.
Kim Seung Yeon
Gyeonggi Business & Science Accelerator
+82 31-776-4834
email us here
Visit us on social media:
LinkedIn
Instagram
Facebook
YouTube
Other
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Construction Mats Market: Growth, Trends, Opportunities & Forecast, 2021 - 2031
'Retired But Ready' Debuts as the Go-To Digital Space for Retirees Seeking Purpose, Connection, and Growth
Vienna Palaces to Coastal Tables: Chef Martin Hoellrigl Redefines Fine Dining
Kalendarium
Więcej ważnych informacji
 Jedynka Newserii
Jedynka Newserii

 Jedynka Newserii
Jedynka Newserii

Prawo

KE proponuje nowy Fundusz Konkurencyjności. Ma pobudzić inwestycje w strategiczne dla Europy technologie
W środę 16 lipca Komisja Europejska przedstawiła projekt budżetu na lata 2028–2034. Jedna z propozycji zakłada utworzenie Europejskiego Funduszu Konkurencyjności o wartości ponad 400 mld euro, który ma pobudzić inwestycje w technologie strategiczne dla jednolitego rynku. Wśród wspieranych obszarów znalazła się obronność i przestrzeń kosmiczna. Na ten cel ma trafić ponad 130 mld euro, pięciokrotnie więcej niż do tej pory.
Firma
Były prezes PGE: OZE potrzebuje wsparcia magazynów energii. To temat traktowany po macoszemu

Choć udział odnawialnych źródeł energii w miksie energetycznym Polski jest stosunkowo wysoki i rośnie, to ten przyrost jest chaotyczny i nierównomiernie rozłożony miedzy technologiami – wskazuje Forum Energii. Dodatkowo OZE potrzebują wsparcia magazynów energii, a zdaniem Wojciecha Dąbrowskiego, prezesa Fundacji SET, ten temat jest traktowany po macoszemu. Brak magazynów powoduje, że produkcja energii z OZE jest tymczasowo wyłączana, co oznacza marnowanie potencjału tych źródeł.
Infrastruktura
Wzrost wynagrodzeń ekip budowlanych najmocniej wpływa na koszty budowy domu. Zainteresowanie inwestorów mimo to nieznacznie wzrasta

Budowa metra kwadratowego domu w Polsce kosztuje od 5,55 do 6 tys. zł w zależności od województwa – wynika z najnowszych analiz firmy Sekocenbud. Najdrożej jest w Warszawie, gdzie cena za metr kwadratowy domu przekroczyła już 6,2 tys. zł. Na przyrosty kosztów budowy domu wpływają zarówno drożejące materiały budowlane, jak i wyższe wynagrodzenia pracowników. Inwestorzy nie rezygnują jednak z budowy domów jednorodzinnych, co ma związek m.in. z wciąż wysokimi cenami mieszkań czy też obniżką stóp procentowych.
Partner serwisu
Szkolenia

Akademia Newserii
Akademia Newserii to projekt, w ramach którego najlepsi polscy dziennikarze biznesowi, giełdowi oraz lifestylowi, a także szkoleniowcy z wieloletnim doświadczeniem dzielą się swoją wiedzą nt. pracy z mediami.











.gif)

 |
| |
| |
|