NETRIS Pharma Announces Positive Interim Phase II Results for NP137 in combination with anti-PD(L)1
Confirmation of NP137 Potential to Overcome Resistance to Immune Checkpoint - Clinical Trial Design and Interim Results to be Presented at ASCO and ESMO 2025
The interim analysis met both the primary and key secondary endpoints in secondary refractory patients. Such analysis confirms NP137 excellent safety profile and its potential to overcome secondary resistance to leading immunotherapies. Clinical benefits were observed in half of the patients with Head & Neck and NSCLC cancers who had previously progressed under approved immunotherapy.
“These interim results mark a major milestone in our mission to develop drugs that overcome therapy resistance,” said Patrick Mehlen, CEO of NETRIS Pharma. “We are excited by the potential of NP137 to restore patients sensitivity to immunotherapy-based treatments and to deliver a new treatment solution for patients with limited options”.
Upcoming Data Presentations
Comprehensive study design and interim results will be presented at two of the world’s leading oncology congresses: the American Society of Clinical Oncology (ASCO) Annual Meeting and the European Society for Medical Oncology (ESMO) Congress in 2025, underscoring the global significance of these findings. “We look forward to sharing these important data with the oncology community at ASCO and ESMO, and to advancing NP137 into the next phase of development,” added Dr. Jérome Fayette, Medical Oncologist at Centre Léon Bérard and Principal Investigator of the ImmunoNET study.
About NP137
NP137 is a humanized monoclonal antibody (IgG1) targeting netrin-1, a protein overexpressed in a large proportion of human cancers and associated with disease severity and resistance to therapy. By blocking netrin-1, NP137 is designed to restore apoptosis and reverse epithelial-to-mesenchymal transition (EMT), addressing critical mechanisms of resistance that limit the effectiveness of immune checkpoint inhibitors. Preclinical and early clinical studies have shown that NP137 has anti-cancer effects both as a monotherapy and in combination with chemotherapy or immunotherapy, with a favorable safety profile.
About NETRIS Pharma
NETRIS Pharma is a clinical-stage biopharmaceutical company focused on developing innovative therapies targeting netrin-1, a protein aberrantly expressed in cancer cells and a key driver of resistance to oncology treatments. The company’s lead product, NP137, is the most advanced netrin-1-targeting candidate and has demonstrated robust anti-cancer activity in both preclinical and clinical settings. NETRIS Pharma is currently conducting four clinical trials: GyNET (NCT04652076), ImmunoNET (NCT05605496), Liver-NET1 (NCT05546879), and LAP-NET1 (NCT05546853).
NETRIS Pharma’s pioneering approach has been recognized by leading European and French organizations, including the EIC Fund, EISMEA, and BPI. The ImmunoNET study is co-funded by the European Union with the EIC Accelerator Program, which supports individual Small and Medium Enterprises (SMEs), in particular Startups and spinout companies to develop and scaleup game-changing innovations.
For more information about NETRIS Pharma, its pipeline, and the ongoing clinical trial, please visit www.netrispharma.com.
Media and Investor Contacts:
NETRIS Pharma
Christophe GUICHARD, CFO and IR,
christopheguichard@netrispharma.com
This press release contains forward-looking statements regarding investigational therapies and clinical development plans. Actual results may differ due to various factors. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or NETRIS Pharma. Neither the European Union nor NETRIS Pharma can be held responsible for them.
Christophe GUICHARD
NETRIS Pharma
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Earth Buddy Offers CBD Products to Keep Pets Calm This Fourth of July
Guardian Recovery - Dallas Addiction Center Now In-Network with Magellan Health Insurance
West Covina High School Esports Team Invited to Prestigious U.S. Navy Rocket League Tournament
Kalendarium
Więcej ważnych informacji
 Jedynka Newserii
Jedynka Newserii

 Jedynka Newserii
Jedynka Newserii

Problemy społeczne
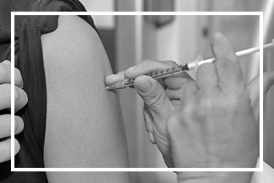
Poziom wyszczepienia Ukraińców jest o 20 pp. niższy niż Polaków. Ukraińskie mamy w Polsce wskazują na szereg barier
Różnice w kalendarzu szczepień, bariery językowe, nieznajomość polskiego systemu szczepień oraz obawy przed skutkami ubocznymi szczepionek – to jedne z najczęstszych problemów, które prowadzą do tego, że poziom wyszczepienia Ukraińców w Polsce jest niższy niż Polaków. To może mieć wpływ na bezpieczeństwo zdrowotne w całym kraju. Fundacja Instytutu Matki i Dziecka podejmuje inicjatywę mającą budować postawy proszczepienne wśród imigrantów.
Polityka
J. Scheuring-Wielgus: Napięcia geopolityczne wymagają silniejszego zjednoczenia państw Europy. To lekcja z II wojny światowej

Parlament Europejski podczas specjalnej sesji w Strasburgu uczcił 80. rocznicę zakończenia II wojny światowej. – Jesteśmy zdeterminowani, by stanąć razem i powiedzieć jasno i stanowczo: nigdy więcej – podkreśliła przewodnicząca PE Roberta Metsola. Zarówno kombatanci, których obecność uświetniła uroczystość, jak i europosłowie podkreślali, że projekt utworzenia europejskiej wspólnoty był odpowiedzią na ówczesne zagrożenia dla pokoju, o który nieustannie trzeba wspólnie dbać.
Ochrona środowiska
Budownictwo modułowe coraz popularniejsze w samorządach. Teraz rozwój sektora jest napędzany przez KPO

Rozwój budownictwa modułowego jest coraz bardziej zauważalny w inwestycjach samorządowych. Wpływa na to przede wszystkim szybsze tempo prowadzonych prac w porównaniu z tymi, w których wykorzystywane są tradycyjne technologie budowlane. Istotną kwestią jest również spełnianie warunków środowiskowych, w tym wysoka efektywność energetyczna budynków, która ma wpływ na możliwość uzyskania dofinansowania z Krajowego Planu Odbudowy. Samorządowcy mają już niewiele czasu na finalizację dotowanych inwestycji. Termin upływa 31 sierpnia 2026 roku.
Partner serwisu
Szkolenia

Akademia Newserii
Akademia Newserii to projekt, w ramach którego najlepsi polscy dziennikarze biznesowi, giełdowi oraz lifestylowi, a także szkoleniowcy z wieloletnim doświadczeniem dzielą się swoją wiedzą nt. pracy z mediami.







.gif)

 |
| |
| |
|