Regenerative Medicine Market to Reach Over USD 403.86 Billion by 2032 with Breakthrough Investments | DataM Intelligence
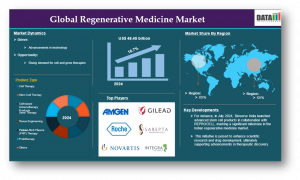
The regenerative medicine market will grow from USD 48.45 Bn in 2024 to USD 403.86 Bn by 2032 at a 27.3% CAGR, supported by recent global investments.
FLORIDA, FL, UNITED STATES, July 30, 2025 /EINPresswire.com/ -- Regenerative medicine encompassing cell therapy, gene therapy, stem cells, tissue engineering, and acellular biomaterials is revolutionizing the treatment of chronic and degenerative diseases. According to DataM Intelligence analysis, the global market size reached USD 48.45 billion in 2024 and is expected to hit USD 403.86 billion by 2032, growing at a 27.3% CAGR from 2025 to 2032. Rising chronic disease prevalence, clinical breakthroughs, and expanded reimbursement are enabling this rapid expansion.Get Your Free Regenerative Medicine Market Sample PDF (Use corporate email ID for Preference): https://www.datamintelligence.com/download-sample/regenerative-medicine-market
(Gain valuable insights into emerging trends, market forecasts, key players, and global investment opportunities.)
Key Players in Regenerative Medicine Market
Notable companies and research institutes shaping the field include:
• bluebird bio, Novartis, Spark Therapeutics, Vertex, Orchard Therapeutics, Gamida Cell, Mesoblast, Gilead, CRISPR Therapeutics – focused on gene and cell therapy pipelines
• Integra LifeSciences, Organogenesis, Allergan, Osiris Therapeutics, Cook Biotech, Medtronic, and Thermo Fisher Scientific – specialized in tissue scaffolds and biomaterial platforms
Academic leaders include the Wake Forest Institute for Regenerative Medicine, known for 3D bioprinted tissues and organ constructs.
Regenerative Medicine Market Segments:
By Therapy Type:
Cell therapy holds the largest market share, driven by established hematopoietic stem cell and CAR-T applications. Gene therapy and tissue engineering are the fastest-growing segments, led by newer gene editing technologies and engineered tissues entering late-stage trials.
By Application Area:
Oncology applications constitute the largest revenue share, owing to high demand for CAR-T cell therapies. Cardiovascular and neurology segments are accelerating fastest, they benefit from unmet needs in heart failure and spinal cord injury, where regenerative interventions show promise.
Take the Next Step—Purchase the Full Regenerative Medicine Market Report: https://www.datamintelligence.com/buy-now-page?report=regenerative-medicine-market
(Get comprehensive data-driven insights, detailed market segmentation, investment trends, and strategic forecasts to support your business decisions.)
Regional Market Dynamics for Regenerative Medicine:
• North America dominates the market, accounting for over 50–58% of global share, thanks to advanced infrastructure, strong regulatory frameworks, and concentrated biotech innovation hubs.
• Asia-Pacific, led by China, Japan, and South Korea, is the fastest-growing region. Japan’s leadership in iPS cell research, regulatory acceleration for regenerative therapies, and coordinated public-private initiatives are significantly driving regional progress.
• South America is an emerging market in regenerative medicine, with Brazil and Argentina leading regional adoption. While infrastructure and regulatory challenges persist, increasing government funding and medical tourism growth are beginning to stimulate clinical research and commercial interest.
• Europe holds a strong share in the global market, backed by supportive ATMP regulations, world-class academic institutions, and major players like Novartis and Lonza. Germany, the UK, and Switzerland are innovation hubs, with rising investments in cell and gene therapy manufacturing.
• Middle East is gaining traction, particularly in GCC countries such as Saudi Arabia and the UAE, where healthcare transformation initiatives and international collaborations are fostering demand for regenerative therapies in orthopedics, aesthetics, and chronic wound care.
Recent Investments & Initiatives
United States
• May 2025: Altaris acquired Minaris Regenerative Medicine and WuXi Advanced Therapies (U.S./U.K. operations), creating Minaris Advanced Therapies, a global CDMO and testing partner for cell therapies.
Japan
• July 2025: JCR Pharmaceuticals was selected under METI’s Regenerative CDMO Subsidy Program, securing funding to modernize facilities, scale its AAV gene therapy platform and expand workforce through 2027.
• July 2025: ReEir, a Japanese regenerative startup, raised ¥580 million (~USD 4.4 million) in pre-Series A funding to advance its cell-based tissue regeneration platforms nationwide.
Europe
• May 2025: In Europe, Coloplast invested USD 1.3 billion in regenerative-related startups, while Bayer and Merck each committed over USD 1.2 billion into cell therapy innovation partnerships part of broader biotech investment strategies across Germany and the UK.
Innovation & Use Cases
• Tissue Bioprinting & 3D Organs: Wake Forest’s ITOP system is enabling lab-grown tissue constructs for wound healing and preclinical models.
• Gene Editing: CRISPR-based therapies targeting hemoglobinopathies and retinal disorders are advancing through clinical pipelines.
• Banking & Tools: Allogeneic cell banking infrastructure is growing fastest as shared off-the-shelf products lower cost and increase scalability.
• Personalized Therapies: Combination products integrating scaffolds, growth factors, and engineered cells support precision treatments in orthopedics and neurology.
Policy & Regulatory Momentum
• Japan pioneered the world’s first fast-track approval framework for regenerative medical products in 2014, allowing outsourced cell culture and expedited product reviews. National agencies such as AMED and METI continue to support CDMO expansion and public private research consortia.
• U.S. FDA and NIH support regenerative trials through RMAT designations and disease-targeted programs, easing commercialization pathways.
Challenges & Future Outlook
Challenges:
• High Manufacturing Costs: Autologous products and complex supply chains drive per-treatment costs into six figures.
• Regulatory Complexity: Scaling from early clinical trials to commercialization requires multi region approvals with stringent quality standards.
• Access & Reimbursement: Broad coverage models and sustainable pricing frameworks are still evolving.
Future Outlook:
With strong momentum in partnerships, CDMO capacity expansion, regulatory modernization and targeted public investment, regenerative medicine is on track to reshape care for chronic diseases, trauma, and degenerative disorders. Growth will be strongest in regions aligning innovation with scalable production particularly in North America, Japan, and Europe poised to propel the market past USD 403.86 Billion by 2032.
Unlock 360° Market Intelligence with 2 Days FREE Trial Access of DataM Subscription Now!: https://www.datamintelligence.com/reports-subscription
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Pipeline Analysis For Drugs Discovery
✅ Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Competitive Landscape
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Related Reports:
Stem Cell Therapy Market Share
Synthetic Biology Market Growth
Sai Kumar
DataM Intelligence 4market Research LLP
+1 877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Inflation Hits Gamers — Incube8 Games Responds with 22% Price Cut on Most Releases
Japan Algae-Based Food Products Market Witnesses Surging Demand Amid Sustainability Trends
Syd Leibovitch, President of Rodeo Realty, Recognized in L.A. Business Journal’s LA 500 for Eighth Year
Więcej ważnych informacji
 Jedynka Newserii
Jedynka Newserii

 Jedynka Newserii
Jedynka Newserii

Problemy społeczne

Ślązacy wciąż nie są uznani za mniejszość etniczną. Temat języka śląskiego wraca do debaty publicznej i prac parlamentarnych
W Polsce 600 tys. osób deklaruje narodowość śląską, a 460 tys. mówi po śląsku. Kwestia uznania etnolektu śląskiego za język regionalny od lat wzbudza żywe dyskusje. Zwolennicy zmiany statusu języka śląskiego najbliżej celu byli w 2024 roku, ale nowelizację ustawy o mniejszościach narodowych i etnicznych zablokowało prezydenckie weto. Ostatnio problem wybrzmiał podczas debaty w Parlamencie Europejskim, ale zdaniem Łukasza Kohuta z PO na forum UE również trudna jest walka o prawa mniejszości etnicznych i językowych.
Transport
Polacy z niejednoznacznymi opiniami na temat autonomicznych pojazdów. Wiedzą o korzyściach, ale zgłaszają też obawy

Polacy widzą w pojazdach autonomicznych szansę na poprawę bezpieczeństwa na drogach i zwiększenie mobilności osób starszych czy z niepełnosprawnościami. Jednocześnie rozwojowi technologii AV towarzyszą obawy, m.in. o utratę kontroli nad pojazdem czy o większą awaryjność niż w przypadku tradycyjnych aut – wynika z prowadzonych przez Łukasiewicz – PIMOT badań na temat akceptacji społecznej dla AV. Te obawy wskazują, że rozwojowi technologii powinna także towarzyszyć edukacja, zarówno kierowców, jak i pasażerów. Eksperci mówią także o konieczności transparentnego informowania o możliwościach i ograniczeniach AV.
Prawo
70 proc. Polaków planuje wyjazd na urlop w sezonie letnim 2025. Do łask wracają wakacje last minute

Ponad 70 proc. Polaków planuje wyjechać na urlop w sezonie letnim, czyli między końcem czerwca a końcem września – wynika z badania Polskiej Organizacji Turystycznej. 35 proc. zamierza wyjechać tylko raz, a 30 proc. – co najmniej dwa razy. Z grupy wyjeżdżających jedna trzecia wybierze się na wyjazd zagraniczny. Jak wskazuje Katarzyna Turosieńska z Polskiej Izby Turystyki, po kilku latach ponownie do łask wracają oferty last minute, a zagraniczne kierunki pozostają niezmienne – prym wiodą m.in. Grecja, Tunezja, Egipt czy Hiszpania.
Partner serwisu
Szkolenia

Akademia Newserii
Akademia Newserii to projekt, w ramach którego najlepsi polscy dziennikarze biznesowi, giełdowi oraz lifestylowi, a także szkoleniowcy z wieloletnim doświadczeniem dzielą się swoją wiedzą nt. pracy z mediami.

![Nestlé w Polsce podsumowuje wpływ na krajową gospodarkę. Firma wygenerowała 0,6 proc. polskiego PKB [DEPESZA]](https://www.newseria.pl/files/1097841585/fabryka-nesquik_1,w_85,r_png,_small.png)






.gif)

 |
| |
| |
|